Being able to access the internet on the go is something most of take for granted these days and the benefits are huge and getting bigger all the time. However, this mobile revolution has been hampered at every step by one key problem, battery power. Big bright screens, quad-core processors, Bluetooth connectivity, and GPS positioning are all great but they're also power-hungry.
Our current devices may seem impressive, but with more power things could change radically. Imagine carrying around your main PC in your pocket all the time, a device with the power of a laptop in the palm of your hand; or possibly a return to when phones needed charging weekly rather than daily. And it's not just phones, tablets and laptops either, as better battery technology could revolutionise electric cars, with bigger ranges and less charging.
What we need is simple – more efficient batteries. To be fair this has already taken place. If smartphones still had nickel cadmium batteries instead of the lithium-ion equivalent, we'd either be talking a battery life of a couple of hours, or they'd be considerably heavier and bulkier. Li-ion batteries have up to five times the energy density of Ni-Cad, and batteries are the largest single component of a phone by weight.
If this doesn't provide you with much consolation, you'll be pleased to hear that scientists are far from finished in their quest for the perfect battery. Here we look at the new battery technologies that are currently in the development labs, at a few high performance equivalents that are already available, and at some alternatives that might even make batteries obsolete.
Assault and Battery
To put it simply, a battery is a device that generates electricity from a chemical reaction. Strictly speaking, what we've just described is a cell while a battery is a number of cells, connected together in a single package, to provide a higher voltage than you'd get from one cell alone. So, for example, an AA alkaline 'battery' is actually a cell and generates 1.5V, while a PP3 battery genuinely is a battery as it contains six 1.5V cells, connected in series, to provide 9V. Despite all this, in deference to common usage, we'll continue to talk about batteries to mean either batteries or cells.
The simplest type of battery is a primary battery and is the type that can't be recharged. As electricity is drawn from the battery, the chemical reaction converts the initial chemicals into other, totally different chemicals. In an alkaline battery, the most common type of primary battery in portable electronics gear, zinc (Zn) reacts with manganese dioxide (MnO2) to produce zinc oxide (ZnO) and a different oxide of manganese (Mn2O3). When there's no zinc or manganese dioxide left, the battery is flat and has to be thrown away.
In a secondary battery, that's a rechargeable one, again a chemical reaction takes place as electricity is produced and, again, when all the original chemicals have been converted to something else, the battery is flat. Where it differs from a primary battery is that the chemical reaction is reversible. To reverse the reaction, electricity is put into the battery instead of taken out of it and this process of recharging causes the original chemicals to be regenerated. The Li-ion battery found in most phones, laptops and tablets converts carbon (C) and lithium cobalt oxide (LiCoO2) into lithium carbide (LiC6) and a different lithium cobalt oxide (LiCo2O4) while electricity is being produced. Exactly the opposite reaction occurs during recharging.
Primary batteries might seem outdated compared to their rechargeable counterparts but they are considerably less expensive and commonly they offer a higher energy density – that's the amount of electrical energy that can be produced for a particular weight or volume of battery – which is why they continue to be used. While the drawback of not being able to recharge a primary battery might be acceptable in something like a toy that is used occasionally, the waste of constantly throwing away batteries would be unacceptable in mobile electronics gear. Because of this, the remainder of this article will concentrate squarely on re-useable energy sources.
Li-ion Roar
It's probably inevitable that the first battery developments that are coming our way would be better described as evolutionary than revolutionary. But it pays not to be dismissive of these gradual changes. After all, look how far LCD has come from simple mono displays to 4K monster TVs, those incremental changes can really add up.
Take Amprius, a start-up company based in Silicon Valley that's commercialising the results of research at Stanford University. Their new battery isn't based on some expensive rare earth element, it doesn't digest microbes, and it doesn't charge in 30 seconds flat. Instead it's based on the lithium-ion technology that's been powering our phones for some time now. But their new spin on the technology allows them to pack an extra 25% of energy into the same volume as an ordinary cell with further improvements in the pipeline. Even with today's Amprius batteries, that's an extra two hours per day and, what's more, this is no pie in the sky research. The battery is already in early prodcution and is being tested by manufacturers including Nokia.

^Lithium-ion batteries might represent a huge leap from NiCads but there's plenty more in the technology yet
So what's the secret of Ampirus' success? It's long been known that using a silicon anode, instead of a carbon one, in lithium-ion batteries, can give a huge performance improvement. This is because four lithium ions will bond to each of the atoms in a silicon anode compared to just one for every six carbon atoms in today's graphite anodes.
This improvement comes at a cost though. Because silicon anodes swell and contract as the battery is charged and discharged, they are soon destroyed so instead of the 500 times today's batteries could be charged, only a few cycles would be possible. However, a team of scientists led by Stanford's Yi Cui has discovered that a double-walled silicon nanostructure will last as long as today's batteries and, potentially, could increase battery life to more than 6,000 cycles.
As Professor Cui described, the new double-walled silicon nanotube anode is made by a clever four-step process: "Polymer nanofibers (green in the diagram) are made, then heated … until they are reduced to carbon (black). Silicon (light blue) is coated over the outside of the carbon fibers. Finally, heating in air drives off the carbon and creates the tube as well as the clamping oxide layer (red)". Because silicon oxide is such a tough ceramic material, the outer oxide layer keeps the outside wall of the nanotube from expanding, so it stays intact.
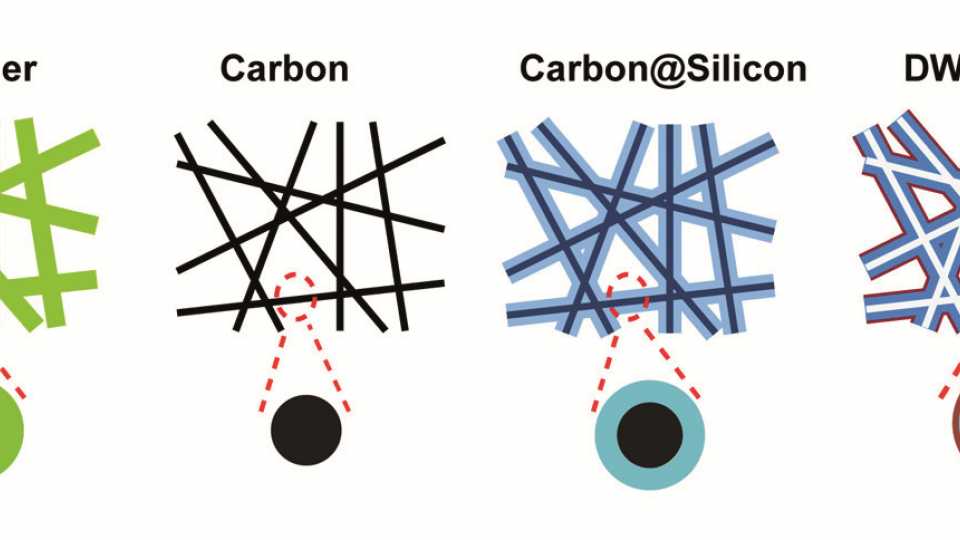
^Anodes made from double-walled silicon nanotubes provide much improved lithium-ion cells (Image: Hui Wu, Stanford, and Yi Cui)
There's even more to come though, with Yi Cui also involved in early work on a lithium anode, which will apparently bring the best possible performance from a Li-ion battery. "Of all the materials that one might use in an anode, lithium has the greatest potential. Some call it the Holy Grail," said Yi Cui. "It is very lightweight and it has the highest energy density. You get more power per volume and weight, leading to lighter, smaller batteries with more power."
Giving examples of how the new battery technology could be used the team said it could increase battery life and bring down costs. Phone battery life could be doubled or tripled and electric cars with a range of 300 miles could cost just $25,000 (£14,700).

